Stoichiometric mixture: What type of mixture it is?
A stoichiometric mixture is a mixture of fuel and air that contains the exact amount of air required for perfect combustion of the fuel. This perfect ratio of fuel and air in the mixture is called the stoichiometric ratio.
This article will shed some light on what a stoichiometric mixture is and how it affects the engine.
Content
What is a stoichiometric mixture?
Each fuel needs a different amount of air for its perfect combustion. For example, with ordinary gasoline (depending on its composition), approximately 14.7 kilograms of air are needed for the perfect combustion of 1 kilogram of this fuel. With diesel, on the other hand, 15 to 15.5 kilograms of air are needed to burn 1 kilogram of fuel perfectly.
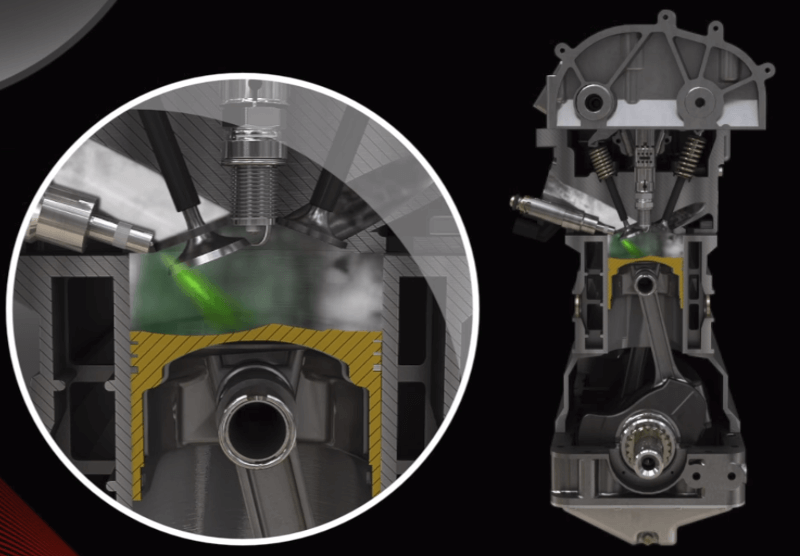
Therefore, if a perfect ratio is maintained between fuel and air in the mixture, the value of the excess air factor will reach 1 (λ = 1). In such a case, we speak of a stoichiometric mixture. If the mixture contains more air, it is called lean; if it contains less air than the stoichiometric ratio, it is called rich.
Stoichiometric mixture and its effect on the engine
Combustion of a stoichiometric mixture is a combustion in which all the oxygen is consumed to burn all the fuel. This is the most optimal combustion we can achieve.

Engine Oil Classification Explained
In theory, emissions should not occur when burning a stoichiometric mixture. In practice, however, the situation is different and due to insufficient homogenization of the fuel and its interaction with other substances such as engine oil, impurities in the fuel or the effect of nitrogen from the air and the short time in which the combustion process must take place, the formation of emissions occurs.
However, since the engines of ordinary cars are mainly operated at partial load, they are designed for this operation so that their operation is as efficient as possible in this mode (lowest fuel consumption and highest ecology).
With this mode of operation of the engine, working with a stoichiometric mixture is a suitable compromise between performance, fuel consumption, and the amount of emissions produced.
In addition, however, the engines of today's cars must first all meet emission limits and so the use of a stoichiometric mixture (λ = 1) seems to be the most suitable because it is during the combustion of a stoichiometric mixture that the catalytic converter has its maximum efficiency and the engine is the most ecological.